More
Epilepsy & Depression
Epilepsy & Depression
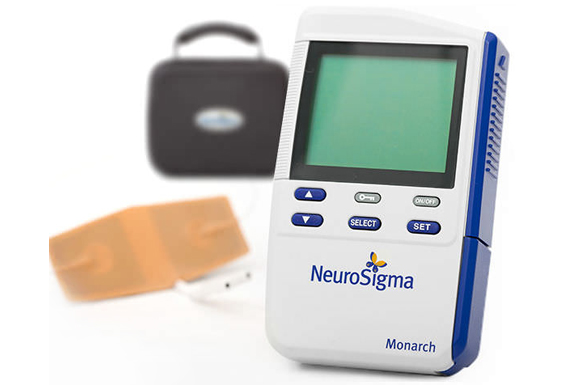


Epilepsy and Depression Treatment Device by Neurosigma
Product Code:
Exploring Innovative Therapies for the future of Neurological Disorders
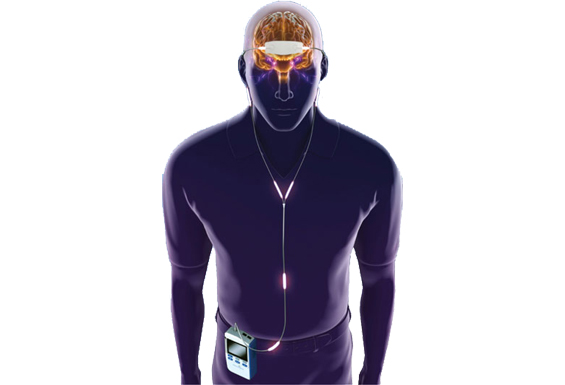
NeuroSigma, Inc. is developing trigeminal nerve stimulation (TNS) for a variety of disorders, including epilepsy, depression, attention deficit hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD) Lennox-Gastaut syndrome (LGS) and traumatic brain injury (TBI). NeuroSigma’s unique TNS technology can be delivered via the non-invasive Monarch external trigeminal nerve stimulation (eTNS™) system or the minimally-invasive subcutaneous trigeminal nerve stimulation (sTNS™) system which is currently under development.
INTRODUCING MONARCH eTNS SYSTEM
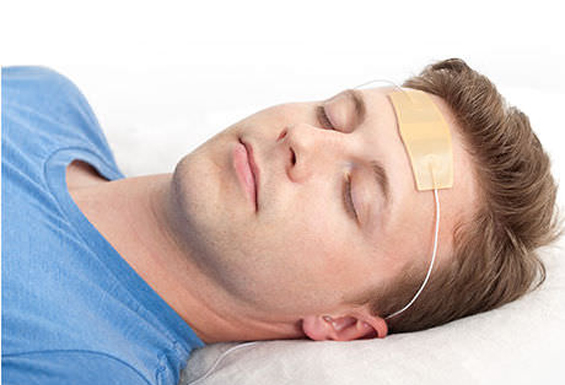
Originally invented at UCLA and developed by a team of doctors and engineers at NeuroSigma, the Monarch eTNS System is a non-invasive medical device that stimulates the trigeminal nerve on the forehead via an external conductive patch. eTNS is a safe and effective medical treatment for ADHD, epilepsy, and depression that can be performed at home, primarily while asleep.
44% REDUCTION IN SYMPTOMS OF ADHD*
eTNS is a new treatment for patients with ADHD that is easy to use and doesn’t have the side effects of medication.
ADHD is one of the most commonly diagnosed conditions in children and is increasingly recognized as an issue that persists into adulthood. Unfortunately, current ADHD treatment is focused on prescription of stimulant medications that can have worrisome side effects.
In a recently-completed study of school age (7-14) children with ADHD, nightly use of eTNS resulted in significant reductions of ADHD symptoms in as little as 4 weeks. Results were similar to those achieved with stimulant medications and patients reported only mild side effects.
52% SYMPTOM REDUCTION IN MAJOR DEPRESSION*
The Monarch eTNS System offers a safe, non-invasive approach for the treatment of major depression, with virtually no side effects.
Depression is a common and disabling medical condition. Many individuals continue to suffer from severe depression despite trying multiple antidepressant medications, often in combination. Few effective options are available to people whose depression is resistant to drug therapies.
Cranial nerve stimulation has been used to treat depression for more than a decade, but the Monarch eTNS System is the first safe and effective system that does not require surgery.
The eTNS system was developed and tested by doctors at the University of California, Los Angeles (UCLA). The eTNS system offers an especially attractive option because it does not require surgery and has been shown to be successful at treating patients with medication resistant depression.
50% OR MORE REDUCTION IN SEIZURES IN 40% OF SUBJECTS*
Separate clinical trials showed the Monarch eTNS System to be a safe and effective treatment for epilepsy.
Treatment of epilepsy with eTNS is easy and convenient.
Patients use the system in the comfort of their own home. Until recently, vagus nerve stimulation (VNS), which requires a surgically-implanted device, was the only cranial nerve stimulation available to epilepsy patients.
The Monarch eTNS system offers a new path for epilepsy patients by providing safe and effective cranial nerve stimulation with a device that does not require surgery.
Physicians at the University of California, Los Angeles (UCLA) invented the eTNS System and separate clinical trials showed it to be safe and effective for treating epilepsy. Patients receiving eTNS also experienced significant improvements in mood and depressive symptoms, making eTNS unique among epilepsy treatments.
*Per clinical trial results
